

 Complaints and Suggestions
Complaints and Suggestions
Tel: +86-755-86913119
E-mail: pro@hku-szh.org
 Consulting and Services
Consulting and Services
Tel: +86-755-86913333
E-mail: info@hku-szh.org
 Address
Address
No.1,Haiyuan 1st Road,Futian District,Shenzhen
 Worktime
Worktime
8:00-17:30 ,Mon. - Sun.(weekend,except public holidays)
The Clinical Trials Center (CTC) of The University of Hong Kong-Shenzhen Hospital is established within the Clinical Trials Center (CTC) as an interdisciplinary, cross-departmental, and independently managed entity with certain administrative functions within the hospital. It provides specialized management and services for Phase I–IV drug clinical trials, medical device clinical trials, and in vitro diagnostic reagent clinical trials at the hospital. The CTC was established in July 2015 and is responsible for the specific management of clinical trials under the guidance of the hospital’s Clinical Trials Center Management Committee (CTC-COM). As a nationally recognized and accredited drug/medical device clinical trial institution (hereinafter referred to as the "Institution"), the hospital authorizes the CTC to perform the functions of the GCP Institutional Office, staffed with dedicated personnel responsible for specific management tasks.
Currently, a total of 27 departments in the hospital are qualified to conduct drug clinical trials, while 39 departments are qualified to conduct medical device clinical trials. The center was approved for the Shenzhen Municipal Development and Reform Commission’s Public Service Platform for Drug Clinical Trials establishment project, with funding of 13 million RMB.
As the first designated pilot medical institution for the "Hong Kong and Macao Drug and Medical Device Access" policy, a national "Province-Ministry Co-constructed High-Quality Development Pilot Hospital," the only medical institution in mainland China to hold both Triple-A Hospital accreditation and ACHS certification, and one of the first high-level hospital construction units in Guangdong Province, the hospital places great emphasis on clinical trials and biopharmaceutical research and development. Drawing on over 20 years of international management experience from The University of Hong Kong Clinical Trials Centre (HKU-CTC) and upholding a strong commitment to trial quality, the hospital is dedicated to developing into a first-class, regionally leading, and internationally renowned one-stop clinical trial management and service platform.
一、National Drug Clinical Trial Institution
The hospital successfully passed the qualification accreditation for the National Drug Clinical Trial Institution (GCP) in May 2017 and the inspection for additional specialties in October 2019. On November 29, 2019, the National Medical Products Administration and the National Health Commission jointly issued the "Regulations on the Administration of Drug Clinical Trial Institutions (No. 101 of 2019)," which changed the GCP qualification accreditation from on-site inspections to a filing system. In response to this change, our hospital proactively took action by thoroughly studying the requirements and actively preparing for the transition. On December 6, 2019, we completed the filing for the drug clinical trial institution, becoming the first hospital in Shenzhen, the third in Guangdong Province, and the fifteenth in China to do so. Currently, there are 27 drug filing specialties available.
Specialties | Respiratory Medicine, Gastroenterology, Neurology, Cardiovascular Medicine, Hematology, Nephrology, Endocrinology, Immunology, General Surgery, Orthopedics, Reproductive Health and Infertility, Neonatology, Pediatric Hematology, Dermatology, Oncology, Gynecology, Phase I Clinical Trial Unit - Phase I Drug Clinical Trials, Phase I Clinical Trial Unit - Bioequivalence Trials, Pediatric Orthopedics, Cardiac and Great Vascular Surgery, Neurosurgery, Thoracic Surgery, Urology, Critical Care Medicine, General Practice, Ultrasound Diagnostics, Anesthesiology |
| National Announcement Website | Drug Clinical Trial Institution Filing Management Information Platform:https://beian.cfdi.org.cn/CTMDS/apps/pub/public.jsp |
二、National Medical Device Clinical Trial Institutions
A total of 39 GCP-filed medical device specialties are available.
Specialties | Respiratory Medicine、Gastroenterology、Neurology、Cardiovascular Medicine、Hematology、Nephrology、Endocrinology、Immunology、General Surgery、Neurosurgery、Orthopedics、Urology、Cardiac and Great Vascular Surgery、Thoracic Surgery、Gynecology、Eugenics、Reproductive Health and Infertility、Neonatology、Pediatric Hematology、Pediatric Orthopedics、Otolaryngology、Stomatology (Dentistry)、Dermatology、Aesthetic Dermatology、Oncology、Anesthesiology、Critical Care Medicine、Clinical Microbiology、Pathology、X-ray Diagnosis、CT Diagnosis、Magnetic Resonance Imaging (MRI) Diagnosis、Interventional Radiology、Radiation Oncology、Traditional Chinese Medicine、Emergency Medicine、Clinical Immunology and Serology、Clinical Chemistry Laboratory、Clinical Body Fluid and Hematology Laboratory |
| National Announcement Website | Medical Device Clinical Trial Institution Filing Management Information Platform:https://beian.cfdi.org.cn/CTMDS/apps/pub/public.jsp |
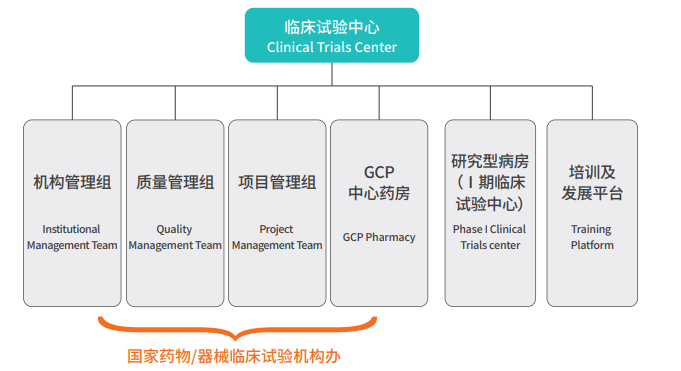
三、Organizational Structure
The center consists of the Institutional Management Group, Quality Management Group, Project Management Group, GCP Central Pharmacy, Phase I Clinical Trial Center, and Training & Development Platform. The organizational structure is as follows:
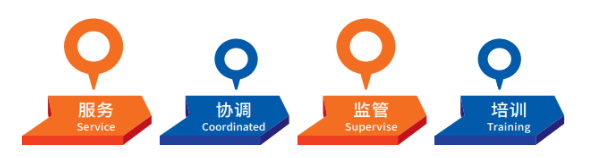
四、Work Responsibilities
The Clinical Trial Center (CTC) is a specialized department within our hospital responsible for the professional management and services of Phase I-IV clinical trials for drugs, medical devices, in vitro diagnostic reagents, and other related studies. Concurrently, it undertakes the duties of the National Drug Clinical Trial Institution Office (referred to as the "Institution Office"). Its responsibilities are organized into four main modules: Service, Coordination, Oversight, and Training.
五、Scope of Business and Services
(一)The primary business management scope of the HKU-SZH CTC includes:
(1) Phase I–IV Drug Clinical Trials
(2) Medical Device Clinical Trials
(3) In Vitro Diagnostic Reagent Clinical Trials
(4) "Hong Kong-Macao Drug and Device Access" Real-World Studies (RWS)
(5) Post-Marketing Re-evaluation Studies Sponsored by Applicants
(二)Provision of One-Stop Clinical Trial Management and Services:
(1) Pre-trial coordination and liaison, feasibility assessment, project initiation management, contract review and management;
(2) Trial support services: centralized drug management, assistance in research plan development, in-hospital CRC services, Phase I clinical trial services, etc.;
(3) Management services during trial implementation: project quality management, drug management, fee management, SMO—Clinical Research Coordinator service management, CRA monitoring management, biological sample management, etc.;
(4) Archival and storage management of research materials;
(5) Professional training (e.g., GCP training, CRC training, study initiation training, etc.).

WeChat official account

Smart Hospital




